1. General
What are the recommended applications for the KAPA RNA HyperPrep Kits?
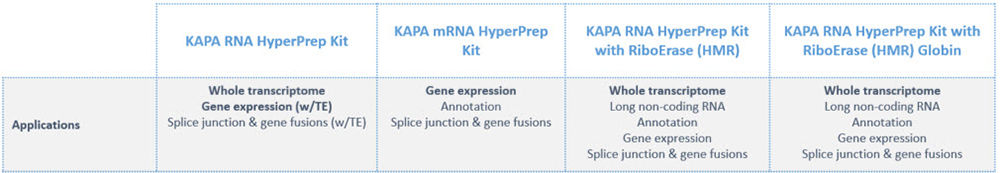
Why would I use rRNA and/or globin depletion over an mRNA capture method?
The poly(A) capture method used in an mRNA capture workflow is useful when specifically interrogating mRNA species, however the workflow does bias towards exonic transcripts. The poly(A) capture approach also tends to have reduced 5' coverage of transcripts, due to the 3' polyadenylation of mRNA transcripts.
The use of the KAPA RNA HyperPrep Kits with RiboErase (HMR) and/or RiboErase (HMR) Globin will allow a more accurate representation of the whole transcriptome and will contain all RNA species except rRNA and/or globin. This workflow retains intronic and intergenic regions, which is where many long non-coding transcripts are found. Additionally, the poly(A) capture approach makes it incompatible for use with degraded RNA, where there is the possibility of strand breaks between the 3' polyadenylated region and the rest of the transcript.
I am using the KAPA Stranded RNA-Seq Kits. Where do I find out more information about these kits?
If you are using one of our KAPA Stranded RNA-Seq Kits, either with mRNA capture or rRNA and/or globin transcript depletion, please visit our online resource for Technical Documentation or alternatively, please visit sequencing.roche.com/contactus.
2. Compatibility
Are these kits compatible with small RNA library preparation?
No, these kits are not compatible with small RNA.
Are these kits compatible with FFPE-derived RNA?
The KAPA RNA HyperPrep Kits (with no upfront enrichment) and the KAPA RNA HyperPrep Kits with RiboErase (HMR) and/or RiboErase (HMR) Globin are compatible with RNA extracted from formalin-fixed paraffin embedded (FFPE) tissue. The quality of FFPE-derived RNA can be highly variable due to the damaging nature of the formalin fixation process, where crosslinking, chemical modification, and fragmentation can occur. Library construction results may vary depending on the input amount and quality of the RNA. Higher RNA inputs may salvage library construction for particularly difficult FFPE samples. Please refer to the recommendations outlined in the Technical Data Sheet for each kit.
KAPA mRNA Hyper Prep Kits are only suitable for mRNA capture and library construction from high-quality input material (RIN ≥ 7). The use of fragmented RNA will result in strong bias towards the 3’-end of the mRNA.

With which species are the KAPA RNA HyperPrep Kits compatible?
The KAPA RNA HyperPrep Kits (with no upfront enrichment) are compatible with any species. The KAPA mRNA HyperPrep Kits are compatible with any species with
3’ poly-A tails.
The KAPA RNA HyperPrep Kits with RiboErase (HMR) or RiboErase (HMR) Globin are compatible with human, mouse, and rat rRNA and/or globin transcript depletion.

Do the KAPA RNA HyperPrep Kits with RiboErase (HMR) and/or RiboErase (HMR) Globin deplete both cytoplasmic and mitochondrial rRNA for human, mouse and rat species?
Yes, both cytoplasmic and mitochondrial rRNA is depleted.
What is the difference between the KAPA RNA HyperPrep Kits with RiboErase (HMR) and the KAPA RNA HyperPrep Kits with RiboErase (HMR) Globin?
Both the KAPA RNA HyperPrep Kit with RiboErase (HMR) and the KAPA RNA HyperPrep Kit with RiboErase (HMR) Globin use the same workflow and reagents, which allows for depletion of rRNA from human, mouse and rat species. The only difference is that an additional tube of depletion oligos are supplied and added during the initial hybridization step of the workflow, to deplete globin transcripts when using the KAPA RNA HyperPrep Kit with RiboErase (HMR) Globin. These globin depletion oligos target globin mRNA derived from hemoglobin alpha 1 (HGA1), hemoglobin alpha 2 (HGA2), hemoglobin beta (HGB), and hemoglobin gamma (HGG).
3. Workflow
What are the major workflow steps in the RNA HyperPrep Kits?
- Fragmentation using heat and magnesium;
- 1st strand cDNA synthesis using random priming;
- Combined 2nd Strand cDNA Synthesis and A-tailing, which converts the cDNA:RNA hybrid to double-stranded cDNA (dscDNA), incorporates dUTP in the second cDNA strand and adds dAMP to the 3′-ends of the dscDNA library fragments;
- Adapter ligation, where dsDNA adapters with 3′-dTMP overhangs are ligated to A-tailed library insert fragments; and
- Library amplification to amplify library fragments carrying appropriate adapter sequences at both ends using high-fidelity, low-bias PCR. The strand marked with dUTP is not amplified, allowing strand specific sequencing.
The upfront enrichment module for mRNA capture make use of magnetic oligo-dT beads. The rRNA and/or globin depletion include hybridization of complementary DNA oligonucleotides, followed by treatment with RNase H and DNase to remove rRNA and/or globin transcripts duplexed to DNA and original DNA oligonucleotides, respectively.
What are the input requirements of the KAPA RNA HyperPrep Kits?

What are KAPA Pure Beads?
KAPA Pure Beads are provided in this kit for reaction purification steps. It is a suspension of paramagnetic beads in a buffer optimized for purification in next-generation sequencing and other molecular biology workflows. KAPA Pure Beads are compatible with manual processing or automated liquid handling and enables efficient recovery in both formats.
Do the kits offer strand-specific information?
Yes, during 2nd strand synthesis, the cDNA:RNA hybrid is converted to dscDNA, with dUTP incorporated into the second cDNA strand. During library amplification, the strand containing dUTP is not amplified, allowing strand-specific sequencing. This kit retains accurate strand origin information in ˃99% of unique mapped reads.
Are there safe stopping points in the sample preparation process?
The sample preparation process may be paused safely after the upfront depletion modules as follows:
After mRNA capture, the resuspended beads (in 22 μL of Fragment, Prime and Elute Buffer) may be stored at 4°C for ≤24 hours.
After rRNA and/or globin depletion, following elution in 1X Fragment, Prime and Elute Buffer, samples may be stored at -20°C for ≤24 hours.
The library construction process from RNA fragmentation through library amplification can be performed in approximately 4 hours, depending on the number of samples being processed, and experience. If necessary, the protocol may be paused safely after any of the following steps:
- After the first post-ligation cleanup, store the resuspended beads at 4°C for up to 24 hours. Do not freeze the beads, as this can result in dramatic loss of DNA.
- After the second post-ligation cleanup, store the eluted, unamplified library DNA at 4°C for ≤1 week, or at -20°C for ≤1 month.
What method of RNA fragmentation does this kit make use of?
RNA is fragmented using high temperature in the presence of magnesium. Depending on the origin and integrity of the input RNA, and the intended application, different RNA fragmentation protocols are provided to obtain the required insert size distribution. For intact RNA, such as that extracted from fresh/frozen tissue, longer fragmentation is required at higher temperatures. For degraded or fragmented RNA (e.g. from older samples or FFPE tissue), use a lower temperature and/or shorter times. The Technical Data Sheet for each product outlines various fragmentation parameters depending on the input RNA and the desired insert size.
What adapters can I use with the KAPA RNA HyperPrep Kits?
KAPA Dual-Indexed Adapters are recommended for use with the KAPA RNA Hyper Prep Kits. However, these workflows are also compatible with other full-length adapter designs wherein both the sequencing and cluster generation sequences are added during the ligation step, such as those routinely used in Illumina TruSeq, SeqCap EZ, Agilent SureSelect XT2, and other similar library construction workflows.
Custom adapters that are of similar design and are compatible with “TA-ligation” of dsDNA may also be used, remembering that custom adapter designs may impact library construction efficiency. Truncated adapter designs, where cluster generation sequences are added during amplification instead of ligation, may require modified post-ligation cleanup conditions. For assistance with adapter compatibility, please contact us.
Where do I find more information about KAPA Dual-Indexed Adapters?
Please refer to the KAPA Dual-Indexed Adapters Technical Data Sheet for information about barcode sequences, pooling, kit configurations, formulation, and dilution for the different KAPA RNA HyperPrep Kits and inputs.
How long can adapter-ligated cDNA be stored?
Purified, adapter-ligated cDNA can be stored at 4°C for one week or at -20°C for at least one month, before amplification and/or sequencing. To avoid degradation, always store DNA in a buffered solution (10 mM Tris-HCl, pH 8.0) and minimize the number of freeze-thaw cycles.
Which polymerase is used for amplification in the KAPA RNA HyperPrep Kits?
KAPA HiFi HotStart DNA Polymerase is the enzyme in the KAPA HiFi HotStart ReadyMix, provided in the KAPA RNA HyperPrep Kits. This is a novel B-family DNA polymerase engineered for low-bias, high fidelity PCR and is the reagent of choice for NGS library amplification1,2,3,4.
- Oyola, S.O. et al. BMC Genomics 13, 1 (2012).
- Quail, M.A. et al. Nature Methods 9, 10–11 (2012).
- Quail, M.A. et al. BMC Genomics 13, 341 (2012).
- Ross, M.G. et al. Genome Biology 14, R51 (2013).
How many cycles should I use when amplifying my adapter-ligated library?
To minimize over-amplification and associated unwanted artefacts, the number of PCR cycles should be optimized to produce enough final, amplified library for downstream analysis and quality control, based on the Illumina instrument requirements. For target capture workflows, typically 1 µg of library yield is required, which may differ depending on the method used and the pre-capture multiplexing strategy employed.
The number of cycles recommended in the Technical Data Sheets for each KAPA RNA HyperPrep Kit should be used as a guide for library amplification, but cycle numbers may have to be adjusted depending on desired final library yield, library amplification efficiency, RNA fragmentation profile, and the presence of adapter dimers.
How should I measure the final library?
The size distribution of the dscDNA and/or final amplified library should be confirmed with an electrophoretic method. The quantification of the library should be performed with a qPCR based quantification kit such as the KAPA Library Quantification Kit for Illumina platforms. This kit employs primers based on the Illumina flow cell oligos, and can be used to quantify libraries that are ready for flow-cell amplification.
4. Data Analysis
What is the best way to analyze my KAPA RNA HyperPrep data?
Roche Sequencing Solutions has partnered with Genialis to provide a data analysis and visualization platform, validated specifically for KAPA RNA HyperPrep Kits. This provides our customers with an end-to-end RNA workflow that is simple, proven and complete. This cloud-based data analysis solution, allows every processing step to be traced to ensure quality and reproducibility and management of data, projects and collaborations.
Benefits of the Genialis platform include:
- Establishing reproducibility across projects by running validated, single-click bioinformatics pipelines through cloud-hosted computational resources so that your primary data analyses are performed the same way across projects.
- Verifying consistency between your experimental replicates and identifying relevant correlations or differences amongst experimental conditions, to ensure design quality.
- Comparison of expression levels across conditions by quantifying gene abundance in individual samples and investigate differential expression of individual genes, pre-defined gene sets, and entire transcriptomes.
- Understand how specific experimental conditions affect the regulation of groups of related genes and their corresponding biological functions.
5. Storage and Quality Control Information
What are the storage conditions for this kit?
The KAPA RNA HyperPrep Kits are supplied in multiple boxes.
- The components for cDNA synthesis and library preparation are temperature sensitive, and should be stored at -15°C to -25°C in a constant-temperature freezer upon receipt. The PEG/NaCl Solution may be stored at 4°C for up to 2 months or at -20°C until expiry date.
- The upfront enrichment module for mRNA capture, should be stored at 2°C to 8°C and for rRNA and/or globin depletion should be stored at -15°C to -25°C upon receipt.
- Store KAPA Pure Beads at 2°C to 8°C.
When stored under these conditions and handled correctly, the kit components will retain full activity until the expiry date indicated on the kit label.
What QC testing is performed on KAPA Adapters?
KAPA Adapters undergo extensive qPCR- and sequencing-based functional and QC testing to confirm:
- optimal library construction efficiency
- minimal levels of adapter-dimer formation
- nominal levels of barcode cross-contamination
Library construction efficiency and adapter-dimer formation are assessed in a low-input library construction workflow. Library construction efficiency is calculated by measuring the yield of adapter-ligated library (before any amplification) by qPCR (using the KAPA Library Quantification Kit), and expressing this as a % of input DNA. To assess adapter-dimer formation, a modified library construction protocol— designed to measure adapter dimer with high sensitivity—is used. Pass criteria for this assay translate to adapter-dimer carry-over in a standard workflow in the range of 0 – 2%.
Barcode cross-contamination is assessed by sequencing. Each adapter is ligated to a unique, synthetic insert of known sequence using a standard library construction protocol. Libraries are pooled and sequenced on a MiSeq™. For every barcode, the number of reads (at least 115,000) associated with each of the 96 inserts is counted, and the total % of correct inserts calculated. Contamination of any barcode with any other single barcode is guaranteed to be <0.25%. The total level of contamination for any barcode is typically in the range of 0.1 – 0.5%. This assay is unable to distinguish between chemical cross-contamination and adapter “cross-talk”, and measures the total number of incorrect inserts resulting from both phenomena. Adapter "cross-talk" occurs when a barcode is "misread" during sequencing and subsequent analysis. The propensity for "cross-talk" differs for different adapter pairs and depends on how closely the barcode sequences are related.
KAPA Adapter Dilution Buffer is free of detectable contaminating exo- and endonuclease activities, and meet strict requirements with respect to DNA contamination.





